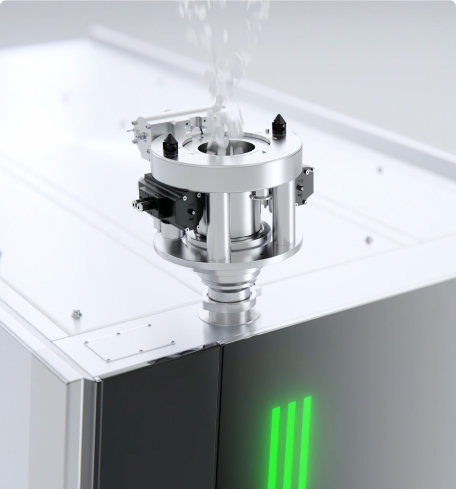
Tablet Coating systems are a central component of validated and safe production processes in the pharmaceutical industry. Active Pharmaceutical Ingredients (API) are protected, release profiles are controlled or the ingestion of tablets is made easier for patients by colour masking or improving swallowing properties. The coating process therefore makes a significant contribution to product quality and therapeutic safety.
In tablet production, Tablet Coaters enable uniform, reproducible coating of tablet cores - regardless of their size or shape. The prerequisite for high-quality tablet coating is an optimised combination of mixing, spraying and drying. This is precisely where the systems from L.B. Bohle come in: We guarantee unrivalled coating uniformity across the market, even with challenging formulations or Active Pharmaceutical Ingredients (API), and ensure the highest coating efficiency with the lowest suspension losses.
Thanks to many years of experience and continuous development, L.B. Bohle's coating systems are among the world's leading technologies in pharmaceutical solids production. Our product portfolio offers systems ranging from Laboratory Coaters for Research & Development or small batches to production-scale Tablet Coaters with batch sizes of up to 1000 litres. L.B. Bohle supplies the optimum solution for every production environment and every scale.

Tablet Coating has various objectives. Release profiles such as enteric-coated, delayed (retarding) or immediate release of the active ingredient can be realised. This ensures that the Active Pharmaceutical Ingredient (API) acts where it is supposed to - and at the desired speed.
Tablet Coating reliably protects the tablet from light, air or moisture, thus extending its shelf life and stability. Tablet coating is also used to neutralise taste or odour or to improve swallowability.
Coloured coatings allow tablets to be clearly distinguished visually and can also be used for advertising purposes.
Tablet Coating systems or Tablet Coaters are indispensable in the production of effective tablets and guarantee a uniform and validatable tablet coating.
Automation
Tablet Coating systems and Tablet Coaters must fulfil the highest requirements in terms of Process Safety, Traceability and Efficiency. Automation is playing an increasingly important role here. All Tablet Coating systems from L.B. Bohle can be integrated into higher-level control systems(MES or SCADA).
This enables, for example, automatic recipe management, real-time reporting and seamless GMP-compliant documentation in accordance with 21 CFR Part 11. Parameters such as drum speed, spray rate, air temperature, air volume and process time are continuously monitored, controlled and documented. This ensures a smooth coating process and achieves the best results.
GMP compliance and Validation
The Tablet Coating systems and Tablet Coaters are designed in accordance with GMP. The GMP standard ensures that pharmaceuticals and Active Pharmaceutical Ingredients (API) are produced with consistent quality. Several processes are validated during Tablet Coating, such as the spray rate, the drying temperature or the drum speed.
Tablet Coater validation ensures that the coating process fulfils the required quality standards. Validation is therefore a decisive criterion for ensuring the quality and consistency of products in the chemical, food and pharmaceutical industries.
Hygienic design and cleaning
Tablet Coating systems or Tablet Coaters have a hygienic design. This ensures that the materials, surfaces and structural elements can be cleaned easily, quickly and completely. With its coating systems, L.B. Bohle favours easy handling and convenient access to all parts.
The Tablet Coaters can be equipped with CIP (Cleaning in Place) or WIP (Washing in Place) to fully automate, validate or document the cleaning process. This ensures maximum safety, especially during product changeovers.
Scalability
A smooth Scale-up from the Laboratory Coater, which is used in Research & Development, to production scale is guaranteed. Batch sizes can be flexibly adapted and the transfer from formulation development to large-scale production batches is efficient and safe.
Flexibility
The Tablet Coaters from L.B. Bohle really come into their own when it comes to difficult formulations and special applications. Special solutions for containment applications for the production of highly active Pharmaceutical Ingredients (API) are increasingly being requested and implemented by the pharmaceutical industry.
The use of a slotted coating drum (slit drum) also offers additional flexibility and efficiency when coating mini-tablets.
The advantages of our coating systems
Highest quality: targeted release of active ingredients, protection and stability, patient acceptance
Automation and Process Safety: Integration into higher-level systems, monitoring and GMP compliance
Hygienic design and easy cleaning: Easily accessible design and effective cleaning (WIP / CIP) ensure fast and valid cleaning
Flexibility: Ideal for demanding formulations, containment applications and customised production
Scalability from the laboratory to production: secure technology transfer thanks to integrated system architecture

{{ product.categoryLabel }}
Weltneuheit
{{ product.title }}
{{ product.link }}
{{ product.subtitle }}
{{ product.description }}
L.B. Bohle offers you a comprehensive range of state-of-the-art machines and systems for your tablet production. Individually tailored to your requirements, our machines optimise your production processes and ensure optimum quality. Our machine finder will guide you quickly and easily to the optimum solution for your processes.
With L.B. Bohle, you can achieve the highest production efficiency and maximum product quality.
High-tech pharmaceutical machinery "Made in Germany". With our extensive product portfolio, we optimise your pharmaceutical solids production. Find out more about the company now.